By Manon Desjardins et Paloma Jacquet
Introduction
Bordetella bronchiseptica is a gram-negative, rod-shaped commensal (and possible pathogen) in many wild and domestic animals (figure 1). It colonizes the respiratory tract and is associated with various lung-related infections. Although many B. bronchiseptica strains possess toxins with the potential to destroy tissue, diseases produced by B. bronchiseptica alone are not always severe. Some diseases can however lead to life-threatening pneumonia. Moreover, infection often predisposes an individual to other infections, some of which can have severe clinical consequences. Although it mainly infects animals, there has been infections in immunocompromised humans and the bacteria is considered zoonotic.

Figure 1: Electron microscopy of B. bronchiseptica biofilm on a glass surface. Source: Nicholson, T.L., Conover, M.S., Deora, R., (2012, November 12). Transcriptome Profiling Reveals Stage-Specific Production and Requirements of Flagella during Biofilm Development in Bordetella bronchiseptica (photograph).
Disease
The main route of transmission for B. bronchiseptica is oral-nasal via direct aerosol droplets (mainly coughing). Infection is initiated by the attachment of B. bronchiseptica to the ciliated cells of the lungs (figure 2A). Inside the lungs, it is able to evade the immune system and create ciliary dysfunction. Ciliated cells have hair-like structure on their surface and are responsible for moving inhaled debris and other pathogens away from the lower respiratory tract. By paralyzing the cilia, B. bronchiseptica increases its chance for colonization and allows for other bacteria to colonize as well. Oftentimes, animals infected with B. bronchiseptica are infected with another bacteria or virus at the same time.
B. bronchiseptica infects a broad range of mammals and gives rise to a wide spectrum of diseases. It is a major cause of “kennel cough” in dogs, which is characterized by persistent, forceful cough, and bronchopneumonia in cats. It is commonly associated with atrophic rhinitis in pigs and snuffles in rabbits. Human disease is rare, but has occurred in individuals that are immunocompromised and occasionally occurs following contact with sick animals. Diseases in this case include pneumonia, sinusitis, and nosocomial tracheobronchitis.
Host symptoms varies depending on the species affected and may include coughing, sneezing, nasal discharge, swelling of the lymph nodes in the neck, lethargy, fever, and difficulty breathing. In severe cases, B. bronchiseptica can be life threatening.
Epidemiology
B. bronchisepticais present and affects animals worldwide. Infections are most commonly found where animals are often in proximity such as animal hospitals, shelters, pet stores and boarding facilities. It is also regularly found in agricultural settings (i.e. commercial rabbiteries) where rapid spread and persistent infection make it difficult to control. Consequent respiratory diseases, which most commonly affects dogs, results in low mortality but morbidity is high. In addition, puppies are much more susceptible than adult dogs because they have yet to develop a strong immune system.
In some situations, the bacteria is present in up to 50% of cat’s nasal swabs from shelters. A European study found that when more cats cohabitate together, more bacteria was isolated from these animals. Another study found that almost 50% of household dogs were carriers of B. bronchiseptica and most of them originated from breeders and pet stores.
Virulence factors
All of B. bronchiseptica‘ s genes for virulence mechanisms are encoded at the bvgAS location in the genome (the genetic material in an organism). Organisms alternate between virulent or non virulent states by turning on or off the bvgAS genes in response to various environmental conditions. Virulence is achieved by causing disfunction in the respiratory tract and the ability to evade the immune system.
Adherence to host cells:
B. bronchiseptica is able to attach to the cells of the upper respiratory system by producing fimbrial adhesins on its surface (figure 2A). They are sticky hair-like structures that allow adherence to host cells and begin infection.
Airway colonization:
The mechanism of ciliated cells paralysis involves the tracheal cytotoxin (TCT) produced by B. bronchiseptica. Ciliated cells in the airways are usually responsible for trapping foreign molecules in a mucus layer and they forcing them out of the body by coughing. TCT targets mitochondria, which produces energy required for the cell’s normal functions. Therefore, it induces ciliostasis meaning it prevents the movement of the cilia. It also causes the extrusion of these cells. Thus, when ciliated cells are paralyzed/killed by this toxin, mucus accumulates in the airways (figure 2B).

Figure 2: Colonization of the ciliated cells by B. bronchiseptica. A) Attachment to the cilia. B) Destruction and paralysis of ciliated cells. Note the mucus that is accumulating in the respiratory tract. Source: Manon Desjardins.
Escape from the immune system:
During a normal immune response, specialized cells such as macrophages and neutrophils migrate to the site of infection and engulf bacteria during a process called phagocytosis (figure 3A) . One of the gene from the bvgAS location encodes for a toxin called adenylate cyclase toxin. The toxin enters the macrophage and/or neutrophil and induces the production of a molecule called cAMP (figure 3B). High cAMP concentration causes metabolic disturbances and the cell is not able to respond to external signals. Therefore, the macrophage/neutrophil cannot phagocytose the bacteria .
B. bronchiseptica also has a type III secretion system. It allows to deliver toxic components from the bacteria directly into host cells by means of a needle-like structure (figure 3C). These molecules, which are called effectors, induce the host cell to commit apoptosis (cell death). Because the molecules are directly deposited into host cells, they avoid being exposed to antibody mediated immune response or to immune cells, hence why this mechanism is important for bacterial survival inside the host.
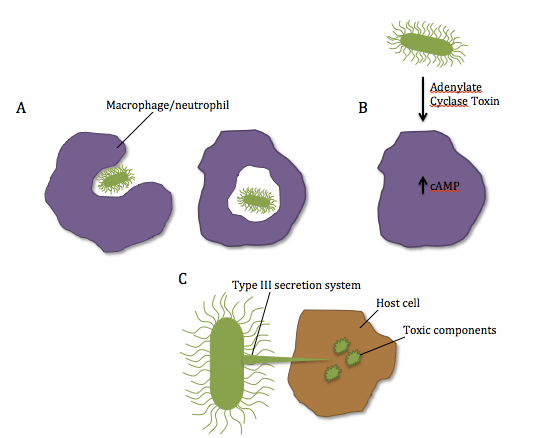
Figure 3: Schematic representation of B. bronchiseptica’s virulence factors. A) Normal mechanism of phagocytosis by neutrophils/macrophages. B) Secretion of Adenylate Cyclase Toxin by the bacteria and inhibition of phagocytosis resulting from elevated cAMP in the cell. C) Direct delivery of toxic components into the host cells with the type III secretion system. Source: Manon Desjardins.
Treatment
In mild cases, the infection can be self-limiting with supportive care. In more severe cases and to treat secondary infections often associated with B. bronchiseptica, antibiotic treatments may be necessary.
Vaccines are available for dogs, cats and swine. Young puppies and kittens may be vaccinated by intranasal, injectable or oral methods. The composition of vaccines depend on the administration method and range from inactivated antigens in injectable vaccines to attenuated avirulent bacteria in intranasal and oral vaccines.
References
Coote, J. G. (2001). Environmental Sensing Mechanisms in Bordetella. Advances in Microbial Physiology, 44, 141-181. Retrieved from http://www.sciencedirect.com/science/article/pii/S0065291101440136
Ford, R. B. (2014). Vital Vaccination Series: Kennel Cough Revisited. Today’s Veterinary Practice. Retrieved from http://todaysveterinarypractice.navc.com/wp-content/uploads/2016/06/T1407C09.pdf
Huebner, E. S., Christman, B., Dummer, S., Tang, Y.-W., & Goodman, S. (2006). Hospital-Acquired Bordetella bronchiseptica Infection following Hematopoietic Stem Cell Transplantation. Journal of Clinical Microbiology, 44(7), 2581–2583. http://doi.org/10.1128/JCM.00510-06
Mattoo, S and Cherry, J. D. (2005). Molecular Pathogenesis, Epidemiology, and Clinical Manifestations of Respiratory Infections Due to Bordetella pertussis and Other Bordetella Subspecies. Clinical Microbiology Reviews, 18(2), 326-382. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1082800/
Nafe, (2014). Diagnostic and Therapeutic Approach: Dogs Infected with Bordetella bronchiseptica & Canine Influenza Virus (H3N8). Today’s Veterinary Practice. 4(4). Retrieved from http://todaysveterinarypractice.navc.com/wp-content/uploads/2016/06/T1407F03.pdf
Nicholson, T.L., Conover M.S., Deora, R., (2012, November 12). Transcriptome Profiling Reveals Stage-Specific Production and Requirement of Flagella during Biofilm Development in Bordetella bronchiseptica (photograph). Retrieved from http://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0049166
Sykes, J. E. (2013). Bordetellosis. Canine and Feline Infectious Diseases (pp. 372-379). Retrieved from http://www.sciencedirect.com/science/book/9781437707953


 Figure 2: A photomicrograph showing the spiral, corkscrew-like shape of Treponema pallidum. The periplasmic flagella allows for the dissemination of the pathogen through host tissues and viscous substances, while preventing recognition by the host defenses. Source: Public Health Image Library, Center for Disease Control, Susan Lindsley (1972).
Figure 2: A photomicrograph showing the spiral, corkscrew-like shape of Treponema pallidum. The periplasmic flagella allows for the dissemination of the pathogen through host tissues and viscous substances, while preventing recognition by the host defenses. Source: Public Health Image Library, Center for Disease Control, Susan Lindsley (1972).
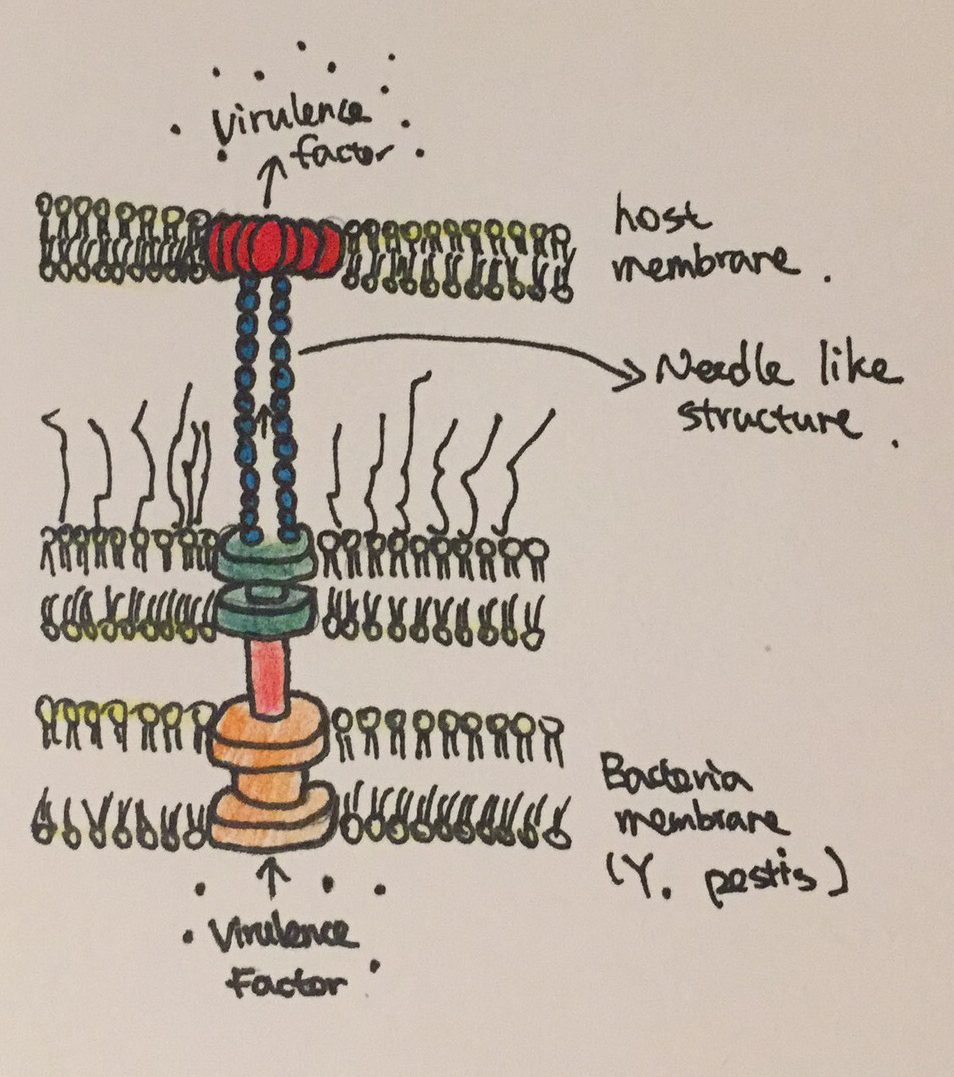 Figure 2: The needle like structure used by Y. pestis to inject virulence factors into host cells.
Figure 2: The needle like structure used by Y. pestis to inject virulence factors into host cells.

 Figure 1: Episcopic differential interference contrast (EDIC) microscopic image of 24-hour exposure showing multi-layered appearance and highly reflective, motile P. mirabilis (‘grey lines’) on silicone catheter section. Source: PLoS ONE, Wilks et al. (2015).à
Figure 1: Episcopic differential interference contrast (EDIC) microscopic image of 24-hour exposure showing multi-layered appearance and highly reflective, motile P. mirabilis (‘grey lines’) on silicone catheter section. Source: PLoS ONE, Wilks et al. (2015).à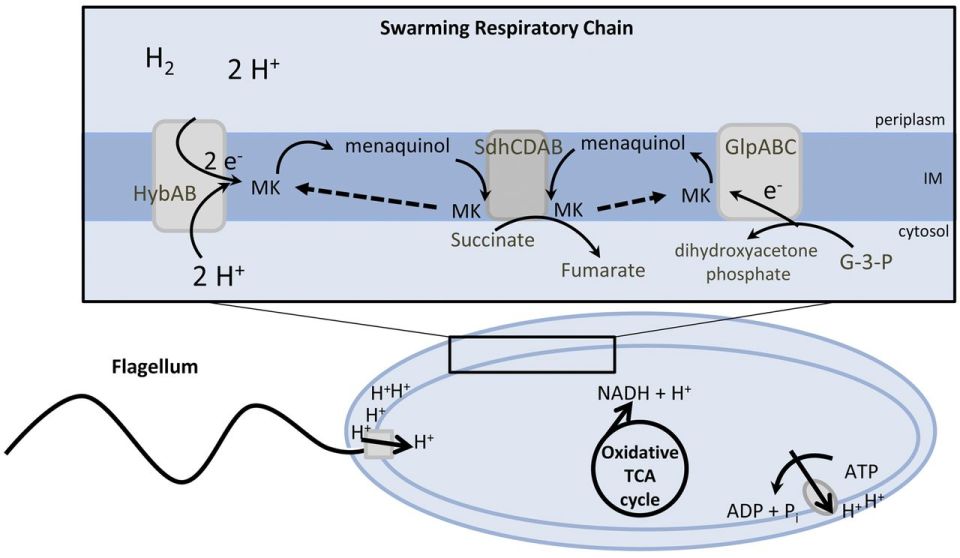 Figure 2: Suggested model for the energy metabolism of P. mirabilis during swarming. The proton gradient is generated by membrane respiration to oxidize NADH to NAD+, which powers flagellum rotation and oxidative phosphorylation. Source: mBio, Alteri et. al (2012).
Figure 2: Suggested model for the energy metabolism of P. mirabilis during swarming. The proton gradient is generated by membrane respiration to oxidize NADH to NAD+, which powers flagellum rotation and oxidative phosphorylation. Source: mBio, Alteri et. al (2012).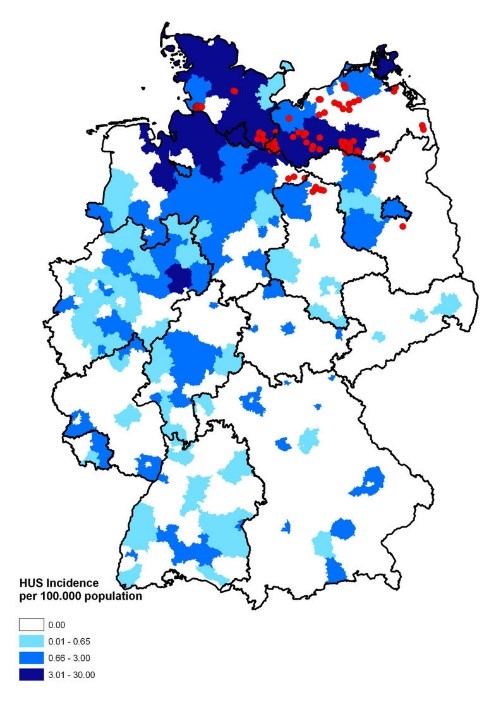 Figure 1: Map of Germany displaying the incidence of HUS [hemolytic uremic syndrome] cases. Source: Wieler LH, Torsten S, Eichhorn I, Antao EM, Kinneman B, Geue L, Karch H, Guenther S, Bethe A. 2011. No evidence of the Shiga toxin-producing E. coli O104:H4 outbreak strain or enteroaggregative E. coli (EAEC) found in cattle faeces in northern Germany, the hotspot of the 2011 HUS outbreak area. Gut Pathogens. [updated 3 November 2011; accessed 17 November 2017];
Figure 1: Map of Germany displaying the incidence of HUS [hemolytic uremic syndrome] cases. Source: Wieler LH, Torsten S, Eichhorn I, Antao EM, Kinneman B, Geue L, Karch H, Guenther S, Bethe A. 2011. No evidence of the Shiga toxin-producing E. coli O104:H4 outbreak strain or enteroaggregative E. coli (EAEC) found in cattle faeces in northern Germany, the hotspot of the 2011 HUS outbreak area. Gut Pathogens. [updated 3 November 2011; accessed 17 November 2017]; 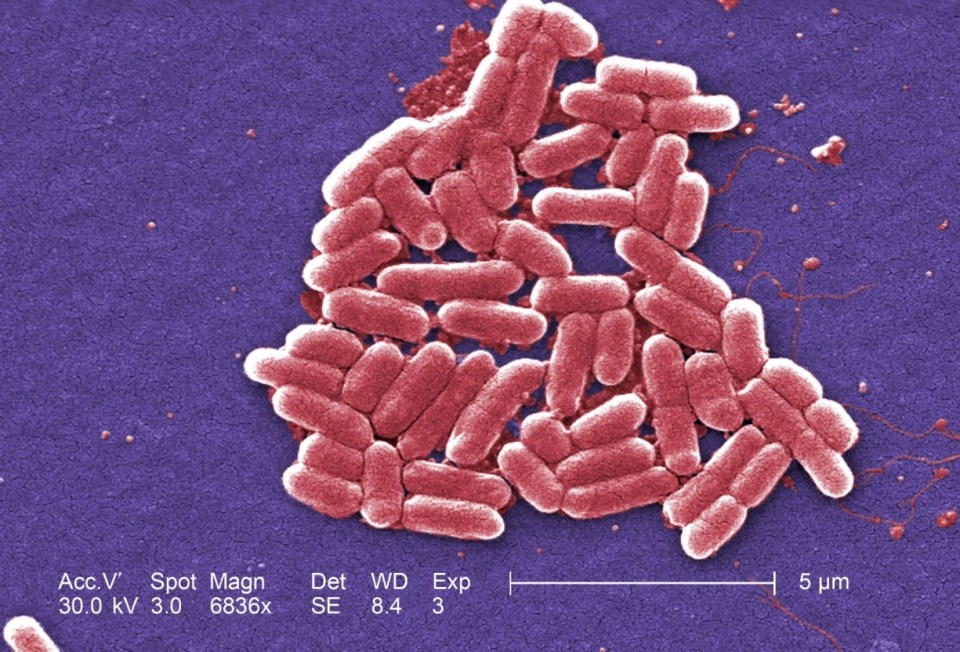 Figure 2: Under a high magnification of 6836X, this digitally-colorized, scanning electron microscopic (SEM) image depicted a growing cluster of Gram-negative, rod-shaped, Escherichia coli bacteria of the strain O157:H7, which is a pathogenic strain of E. coli. Source: Public Health Image Library, Center for Disease Control, Dr. Janice Carr (2006).
Figure 2: Under a high magnification of 6836X, this digitally-colorized, scanning electron microscopic (SEM) image depicted a growing cluster of Gram-negative, rod-shaped, Escherichia coli bacteria of the strain O157:H7, which is a pathogenic strain of E. coli. Source: Public Health Image Library, Center for Disease Control, Dr. Janice Carr (2006).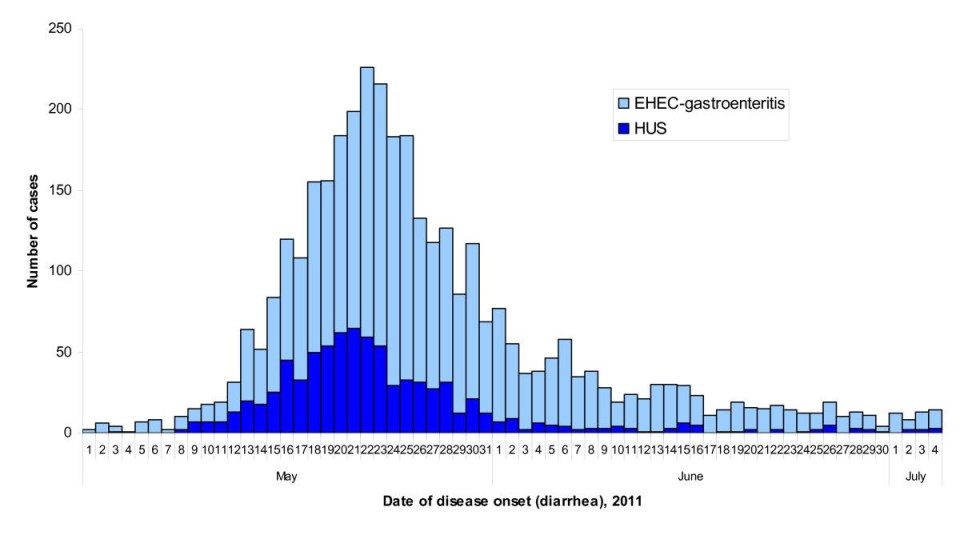 Figure 3: Epidemic curve of the 2011 outbreak of O104:H4 strain of Escherichia coli in Europe. Source: Werber D, Krause G, Frank C, Fruth A, Flieger A, Mielke M, Schaade L, Stark K. 2012. Outbreaks of virulent diarrheagenic Escherichia coli- are we in control? BMC Medicine. [accessed 2017 Nov 17];
Figure 3: Epidemic curve of the 2011 outbreak of O104:H4 strain of Escherichia coli in Europe. Source: Werber D, Krause G, Frank C, Fruth A, Flieger A, Mielke M, Schaade L, Stark K. 2012. Outbreaks of virulent diarrheagenic Escherichia coli- are we in control? BMC Medicine. [accessed 2017 Nov 17]; 

 Figure 1: The characteristic buboes of the bubonic plague, seen here in a man’s armpit. The bubo is circled in red. Source: Centre for Disease Control and Prevention. 2017.
Figure 1: The characteristic buboes of the bubonic plague, seen here in a man’s armpit. The bubo is circled in red. Source: Centre for Disease Control and Prevention. 2017.