by Malak Sadek and Rana Elyamany
Introduction
Neisseria gonorrhoeae is the causative agent of the sexually transmitted infection gonorrhea which is is the second most prevalent bacterial sexually transmitted infection worldwide. Its formal identification was in 1879 by the German bacteriologist Albert Neisser. Gonorrhea grows mainly in the warm, moist areas of the reproductive tract for both men and women. It can also grow in the mouth, throat, eyes, and anus.
Disease
Neisseria gonorrhoeae infections are acquired in humans by sexual contact. It is able to infect the lower genital tract, urethra in men and cervix in women (see Figure 1 and 2). Infected women may be asymptomatic (show no symptoms), but up to 50% show nonspecific symptoms including odorless mucopurulent, vaginal discharge and vaginal bleeding. Even infections without symptoms can also result in severe consequences. On the other hand, 90% of men with urethral infection have symptomatic mucopurulent penile discharge and dysuria. Gonococci can ascend to the upper genital tract, leading to serious diseases, such as epididymitis in men and cervicitis, endometriosis, and pelvic inflammatory disease in women. When N. gonorrhoeae infects the urogenital tract, it interacts with a variety of cells, including Polymorphonuclear leukocytes (PMNs or neutrophils). PMNs are professional cells of the immune system which often serve as the first line of host defense against the bacterial infection. N. gonorrhoeae can survive and replicate inside PMNs and this interactions likely play a critical role in the pathogenesis of infection (see Figure 3).

Figure 1: This illustration depicts a urethral exudate containing Neisseria gonorrhoeae from a patient with gonococcal urethritis. N. gonorrhoeae appears as typical intracellular (pink) diplococcic. Source: Public Health Image Library, Center for Disease Control. Dr. Norman Jacobs (1974).

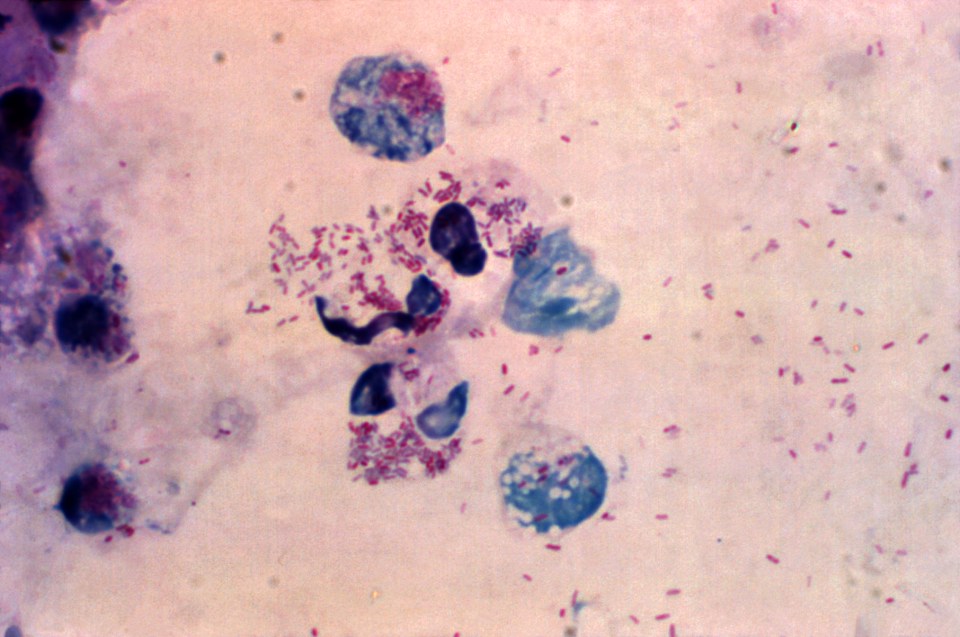
Figure 2: This illustration depicts Neisseria gonorrhoeae in a cervical smear using the Gram-stain technique.. N. gonorrhoeae appears as typical intracellular (pink) diplococcic. Source: Public Health Image Library, Center for Disease Control. Dr. Joe Miller (1975)
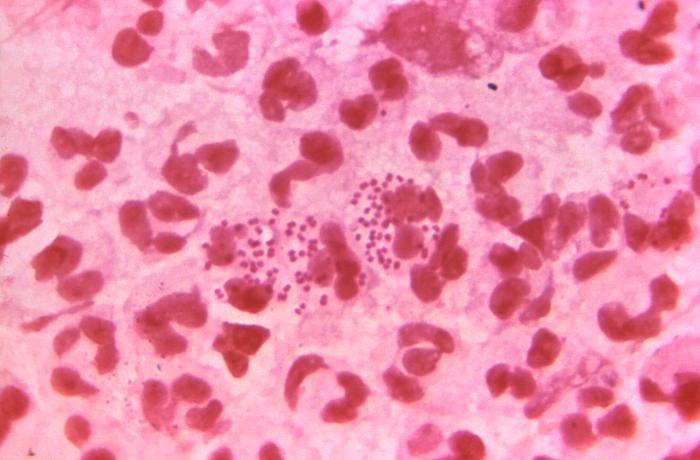
Figure 3: Microscopic image the presence of intracellular Neisseria gonorrhoeae amongst numerous white blood cells (WBCs) known as polymorphonuclear leukocytes, or PMNs. N. Gonorrhoeae cells are pink diplococcal. Source: Public Health Image Library, Center for Disease Control. Dr. Bill Schwartz (1971).
Epidemiology
Gonorrhea is a very common infectious disease. It is the second most prevalent sexually acquired infection in the United States, with more than 300,000 reported cases per year. The Center for Disease Control and Prevention (CDC) estimates that annually more than 700,000 people in the United States get new gonorrhea infections. The most recent data provided by the (CDC) indicate that reported cases have increased by almost 10% over the last 5 years. In 2013, 106.1 cases of N. gonorrhoeae per 100,000 persons were reported, representing an 8.2% increase in incidence from 2009. Approximately 75 percent of all reported cases of gonorrhea are found in younger persons 15 to 29 years of age. The prevalence of this infection has strong economic effects, as in 2008, the total lifetime direct medical cost of N. gonorrhoeae infections in the United States was estimated to be $162.1 million.
Virulence systems
Neisseria gonorrheae express a set of common mechanisms that allow its adaptation to the immune system and immune evasion. .Gonorrheae first attaches itself to the cells and the tissues using a rod shaped protein structure called Type IV pili that recognizes a molecule of the host and bind to it (the host is the human in whom the bacteria live and cause infection). Therefore Type IV pili is essential for virulence in N. gonorrhoeae because it mediates specific attachment to human mucosal cells, initiating the infectious process. It is known that type IV pili is formed from subunits of a protein called pilin, and a gene called pilE in the genome of N. gonorrhoeae, is responsible for encoding the pilin subunit. Notably, Type IV pili in gonorrhoeae is a surface antigen, an antigen is a substance that causes the immune system to produce large proteins called antibodies to identify and neutralize the bacteria. Because N. gonorrhoeae undergoes antigenic variation, the capacity to generate much more diversity in its surface antigens, it has the ability to resist the killing cells in the host. A mechanism called gene shuffling or gene conversion allows N. gonorrhoeae undergoes antigenic variation events.Through DNA recombination (DNA exchange between genes),pilE gene can exchange a part of itself with pilS gene copy. Subsequently a new variant of pilE protein will be produced which is not the same as the original one (see Figure 4). Producing different types of pili increases the chance of evading the immune system especially the antibodies response. Antibodies should bind to adhesions such as typeIV pili and prevent the attachment of the bacteria to the host cells. Furthermore antibodies increase the attachment of the bacteria to phagocytes (immune system cells that pick up and kill the bacteria by a process called phagoctosis) .Therefore, it is assumed that antibodies reacting with pili type IV could block the infection. However, switching of pili expression states alters bacterial interactions with host cells. Thus varying the antigens that are presented to the host immune system prevents antibodies binding to the bacterial surface and subsequently phagocytosis.

Figure 4: Antigenic variation in Neisseria gonorrhoeae by gene shuffling. The white boxes represent the conserved regions of pilE and pilS. The variable sequences (mc1-mc6) are represented by the yellow boxes for pilS and the pink boxes for pilE. Sma/Cla is DNA sequence that is involved in pilin recombination.
Treatment
N. gonorrhoeae has developed resistance to mainly all antibiotics introduced for treatment of gonorrhea. These drugs include: cefixime (an oral cephalosporin), ceftriaxone (an injectable cephalosporin), azithromycin, and tetracycline. N. gonorrhoeae is able to use number of mechanisms for antibiotics resistance including enzymes to degrade antibiotics. Recently, Centers for Disease Control and Prevention (CDC) recommends only ceftriaxone plus either azithromycin or doxycycline as first-line treatment for gonorrhea.
References:
Hill, S. A., & Davies, J. K. (2009). Pilin gene variation in Neisseria gonorrhoeae: reassessing the old paradigms. FEMS Microbiology Reviews, 33(3), 521–530.
JANET A. M. FYFE, C.S.C., AND JOHN K. DAVIES. 1995. The pilE Gene of Neisseria gonorrhoeae MS11 Is Transcribed from a s70 Promoter during Growth In Vitro. JOURNAL OF BACTERIOLOGY 177, No. 13: 3781–3787.
Lancaster et al (2015) Update on Treatment Options for Gonococcal Infections. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy.
Rotman and seifet (2014) The Genetics of Neisseria Species. Annual Reviews 48: 405-431. doi: 10.1146/annurev-genet-120213-092007.
Seifert, A.K.C.a.H.S. 2012. A bacterial siren song: intimate interactions between Neisseria and neutrophils. Nature Reviews Microbiology 10, 178-190. doi: 10.1038/nrmicro2713.
Unemo, M., & Shafer, W. M. (2011). Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Annals of the New York Academy of Sciences, 1230, E19–E28. http://doi.org/10.1111/j.1749-6632.2011.06215.x



 So just what is anthrax? To go textbook, it’s an aerobic, gram-positive (Fig 2), spore-forming bacteria that is naturally found worldwide in soil, with a preference for growing in warm, wet climates [1, 2]. That means that when conditions
So just what is anthrax? To go textbook, it’s an aerobic, gram-positive (Fig 2), spore-forming bacteria that is naturally found worldwide in soil, with a preference for growing in warm, wet climates [1, 2]. That means that when conditions  are suboptimal, anthrax can change from an actively replicating, rod shaped bacteria into a dormant endospore form, which can resist drying out, extreme heat, cold temperatures, ultraviolet light and disinfectants [1, 2]. Anthrax is a zoonotic bacteria that mainly affects the hoofed animal, but can be transmitted to humans through contaminated animal products [2].
are suboptimal, anthrax can change from an actively replicating, rod shaped bacteria into a dormant endospore form, which can resist drying out, extreme heat, cold temperatures, ultraviolet light and disinfectants [1, 2]. Anthrax is a zoonotic bacteria that mainly affects the hoofed animal, but can be transmitted to humans through contaminated animal products [2].
 inhalational anthrax are the three traditional modes of transmission. Your whole body is basically designed to try and fight this kind of thing, but anthrax is a really resourceful attacker. The body’s immune response does its best to protect you from these invaders, but it is no match for the anthrax spore [2].
inhalational anthrax are the three traditional modes of transmission. Your whole body is basically designed to try and fight this kind of thing, but anthrax is a really resourceful attacker. The body’s immune response does its best to protect you from these invaders, but it is no match for the anthrax spore [2]. Virulence factors allow a microorganism to cause disease, and anthrax has some good ones. It uses a combination of a capsule and potent exotoxins to evade and destroy. The capsule forms a protective shell around the growing bacteria, allowing it to get into host cells and use them like a taxi cab to travel to your lymph nodes, where it can spread through the blood [2].
Virulence factors allow a microorganism to cause disease, and anthrax has some good ones. It uses a combination of a capsule and potent exotoxins to evade and destroy. The capsule forms a protective shell around the growing bacteria, allowing it to get into host cells and use them like a taxi cab to travel to your lymph nodes, where it can spread through the blood [2].![Figure 3: Anthrax releases its exotoxins (Fig 3.), the lethal factor and the edema factor, into the body. The protective antigen basically acts as a kind of Trojan horse, sitting on the surface of your body’s cells and smuggling them in to where they can do their damage. Edema factor makes the cell swell with water, while the lethal factor (again, scientists with their creative naming tactics) kills the cell by rupturing its membrane through a process called lysis. Similarly to how you don’t do too well when overfull or full of holes, neither do your cells, which is what makes anthrax such a dangerous player [2]. (Image by C. Mastromonaco).](https://mechpath.com/wp-content/uploads/2015/12/figure3.png?w=960)
 Bear with us here: we’re going to look at some numbers, compiled in a 2014 issue of Eurosurveillance. Injectional anthrax has exhibited what’s called a bimodal distribution: it first presented itself en masse in December 2009 (having only cropped up previously in a single case in the year 2000), and was followed by a second cluster of cases in June 2012. From 2009-2010, 126 cases of anthrax contracted by heroin users were reported, 95% of which were diagnosed in bonnie Scotland, which unfortunately doesn’t seem to be able to add “pure, unadulterated narcotics” to its list of tourist attractions. Between 2012 and 2013, 15 more cases have emerged in a half a dozen different European countries A 33% mortality rate was reported the first go-around, but the fatality in this more recent wave is much higher, with an estimated 47% of cases resulting in death (Fig. 4).
Bear with us here: we’re going to look at some numbers, compiled in a 2014 issue of Eurosurveillance. Injectional anthrax has exhibited what’s called a bimodal distribution: it first presented itself en masse in December 2009 (having only cropped up previously in a single case in the year 2000), and was followed by a second cluster of cases in June 2012. From 2009-2010, 126 cases of anthrax contracted by heroin users were reported, 95% of which were diagnosed in bonnie Scotland, which unfortunately doesn’t seem to be able to add “pure, unadulterated narcotics” to its list of tourist attractions. Between 2012 and 2013, 15 more cases have emerged in a half a dozen different European countries A 33% mortality rate was reported the first go-around, but the fatality in this more recent wave is much higher, with an estimated 47% of cases resulting in death (Fig. 4).![Figure 4: This beautiful, color coded graph breaks down the timing and geography so that we don’t have to! You can see the two different clusters of disease presentation, and the different countries that were affected [6]. (Source: Eurosurveilance)](https://mechpath.com/wp-content/uploads/2015/12/figure4.jpg?w=960)
 uncommon, it doesn’t seem to elicit any of the standard markers for inflammation, like a higher number of white blood cells or elevated levels of C-reactive protein (CRP). It’s pretty weird – they suspect that it’s tied to the more immediate action of
uncommon, it doesn’t seem to elicit any of the standard markers for inflammation, like a higher number of white blood cells or elevated levels of C-reactive protein (CRP). It’s pretty weird – they suspect that it’s tied to the more immediate action of  the edema factor within the tissue, as it doesn’t have to cross the regular barriers [7]. So essentially, injectional anthrax presents most frequently as a kind of deep-tissue cutaneous anthrax with skin infection and blistering, but without any of the helpful markers for the disease that doctors look for. This, along with anthrax’s current state of “how on Earth would you get exposed to THAT?” led to some confusion and misdiagnosis, as the symptoms present similarly to a few other diseases.
the edema factor within the tissue, as it doesn’t have to cross the regular barriers [7]. So essentially, injectional anthrax presents most frequently as a kind of deep-tissue cutaneous anthrax with skin infection and blistering, but without any of the helpful markers for the disease that doctors look for. This, along with anthrax’s current state of “how on Earth would you get exposed to THAT?” led to some confusion and misdiagnosis, as the symptoms present similarly to a few other diseases.
 anthrax often results in one of the most terrifying kinds of inflammation: meningitis (Fig. 5). There are three layers called the meninges wrapped around your brain and spinal cord. Large numbers of bacteria between the layers spells bad news for your brain, and in the case of anthrax, meningitis kills about 96% of the time [5]. Once the bacteria has gone systemic, it’s easy to see why it appeals to strongly to those in the heavy metal profession: the presence of large quantities of anthrax bacilli in your blood stream turns your blood so dark that it appears black.
anthrax often results in one of the most terrifying kinds of inflammation: meningitis (Fig. 5). There are three layers called the meninges wrapped around your brain and spinal cord. Large numbers of bacteria between the layers spells bad news for your brain, and in the case of anthrax, meningitis kills about 96% of the time [5]. Once the bacteria has gone systemic, it’s easy to see why it appeals to strongly to those in the heavy metal profession: the presence of large quantities of anthrax bacilli in your blood stream turns your blood so dark that it appears black.
 Turkey, but this is unlikely to be the case. Genetically analyzing all available isolated strains (or isolates) of injectional anthrax suggests that there were at least two separate contamination events – one for each outbreak – as there are two different genetic clusters of anthrax bacilli. They’re not related closely enough on the anthrax family tree to have come from the same source, and according to their genetic makeup, they branched off at completely different times. The evidence does suggest that they come from the same country, but this is not necessarily true [9].
Turkey, but this is unlikely to be the case. Genetically analyzing all available isolated strains (or isolates) of injectional anthrax suggests that there were at least two separate contamination events – one for each outbreak – as there are two different genetic clusters of anthrax bacilli. They’re not related closely enough on the anthrax family tree to have come from the same source, and according to their genetic makeup, they branched off at completely different times. The evidence does suggest that they come from the same country, but this is not necessarily true [9].